Background: Patients with RRMM receive multiple lines of therapy in their disease course and often become refractory to or are intolerant of newer approved therapies (eg, IMiDs, anti-CD38 monoclonal antibody [mAb] therapy, proteasome inhibitors [PIs]), limiting effective treatment options (Kumar et al. Leukemia. 2017; 31:2443). Efficacy outcomes decrease with each line of therapy, showing a need for agents with novel mechanisms of action to improve treatment responses and survival outcomes (Gandhi et al. Leukemia. 2019;33:2266).
Melphalan flufenamide (melflufen) is a first-in-class peptide-drug conjugate (PDC) that targets aminopeptidases and rapidly releases alkylating agents into tumor cells. In the phase 2 HORIZON study (OP-106; NCT02963493), melflufen plus dexamethasone demonstrated encouraging efficacy in heavily pretreated patients with RRMM refractory to pomalidomide and/or an anti-CD38 mAb (overall response rate [ORR], 29%; median progression-free survival [PFS], 4.2 months; median overall survival [OS], 11.6 months) with a clinically manageable safety profile characterized primarily by hematologic adverse events (Richardson et al. EHA 2020. Abs. EP945).
A contemporary representative control cohort is often used to indirectly compare outcomes of patients in clinical trials versus real-world data. The retrospective MAMMOTH study investigated the natural history and outcomes of patients with RRMM refractory to anti-CD38 mAb therapy after treatment with conventional agents (Gandhi et al. Leukemia. 2019;33:2266), providing a benchmark for the evolving state of the RRMM treatment landscape. Outcomes in clinical studies of novel agents in RRMM have been compared with MAMMOTH previously (Costa et al. ASH 2019. Abs. 3125), but not specifically in patients with RRMM refractory to anti-CD38 mAb therapy.
To contextualize the clinical benefit of melflufen plus dexamethasone compared with conventional agents for patients with RRMM refractory to anti-CD38 mAb therapy, an analysis was conducted evaluating efficacy outcomes in HORIZON versus MAMMOTH.
Methods: HORIZON patients with RRMM refractory to anti-CD38 mAb in the last line of therapy in the intention-to-treat population at final analysis (data cutoff date, 14 Jan 2020) were included in this analysis (N=63). Patients received melflufen 40 mg (intravenously on day 1 of each 28-day cycle) plus dexamethasone 40 mg weekly until disease progression or unacceptable toxicity. The MAMMOTH dataset included patients who progressed while on anti-CD38 mAb therapy (N=275) and were subsequently treated (N=249). ORR was assessed by the investigator per the International Myeloma Working Group criteria. PFS was defined as the time between the first dose of therapy and disease progression or death. OS was defined as the time from refractoriness to anti-CD38 mAb therapy to death from any cause in contrast to clinical studies in which OS is generally measured from first dose of subsequent therapy.
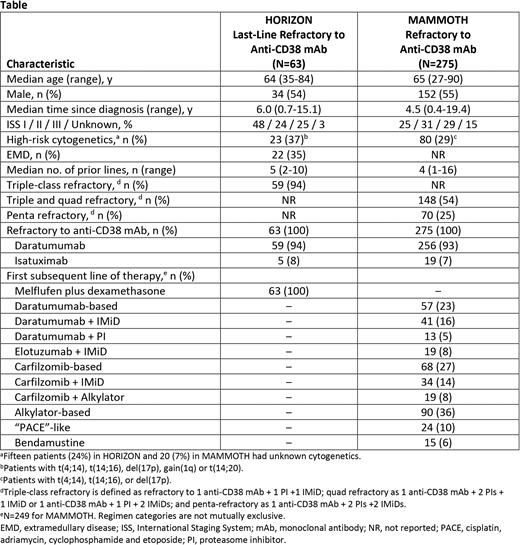
Results: Patients in HORIZON and MAMMOTH had similar baseline median age and profile of daratumumab and isatuximab refractoriness (Table). Patients in HORIZON were more heavily pretreated than those in MAMMOTH (median number of prior therapies [range], 5 [2-10] versus 4 [1-16]). Patients in MAMMOTH were often treated with triplet therapy as first subsequent therapy after anti-CD38 mAb therapy failure (Table). ORR for HORIZON patients who failed last-line therapy with an anti-CD38 mAb (N=63) was 35% versus 31% for all anti-CD38 mAb therapy-refractory patients who received conventional agents in MAMMOTH. Median PFS was 4.6 months (95% CI, 3.7-6.4) versus 3.4 months (95% CI, 2.8-4.0), and median OS from last line was 15.4 months (95% CI, 12.0-25.6) versus 9.3 months (95% CI, 8.1-10.6) for these patients in HORIZON and MAMMOTH, respectively.
Conclusion: This indirect comparison of patients with RRMM refractory to anti-CD38 mAb therapy in HORIZON and MAMMOTH suggests longer survival outcomes with melflufen plus dexamethasone (median PFS and OS of 4.6 and 15.4 months, respectively) versus those with conventional agents, despite HORIZON having more heavily pretreated patients. This analysis underscores the high burden of disease and unmet needs for patients with RRMM and the feasibility of novel combination therapy in this setting.
Blade Creixenti:Takeda: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. Mateos:Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Honoraria; Regeneron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive: Honoraria, Membership on an entity's Board of Directors or advisory committees. Oriol:GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; Amgen: Consultancy, Speakers Bureau; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Larocca:GSK: Honoraria; Takeda: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria. Cavo:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel accomodations, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel accomodations, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GlaxoSmithKline: Honoraria, Speakers Bureau; Karyopharm: Honoraria; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Rodríguez-Otero:Janssen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Abbvie: Consultancy; Kite: Consultancy; Sanofi: Consultancy; Celgene/Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Medscape: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; GlaxoSmithKline: Consultancy, Current Employment, Current equity holder in publicly-traded company; Oncopeptides: Consultancy. Leleu:GSK: Honoraria; Sanofi: Honoraria; Novartis: Honoraria; Carsgen: Honoraria; Incyte: Honoraria; Merck: Honoraria; Amgen: Honoraria; Karyopharm: Honoraria; AbbVie: Honoraria; BMS-celgene: Honoraria; Janssen: Honoraria; Oncopeptide: Honoraria. Nadeem:Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Adaptive: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Janssen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES. Hassoun:Novartis: Consultancy; Celgene: Research Funding; Takeda: Research Funding. Touzeau:Abbvie: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Amgen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; GlaxoSmithKline: Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Sanofi: Honoraria, Research Funding. Amor:Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene-BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; GSK: Membership on an entity's Board of Directors or advisory committees. Maisel:Takeda: Honoraria, Speakers Bureau; Incyte: Honoraria, Speakers Bureau; Kite: Honoraria, Speakers Bureau; Texas Oncology: Current Employment; Karyopharm: Honoraria, Speakers Bureau; Incyte: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Karyopharm: Honoraria, Speakers Bureau; Texas Oncology: Current Employment; Celgene: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Kite: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; Celgene: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau. Mazumder:The Oncology Institute: Current Employment; Amgen: Honoraria, Speakers Bureau; Celgene: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau. Raptis:INTEGRA: Consultancy, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; UPMC: Current Employment. Puig:BRISTOL-MYERS SQUIBB: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Research Funding, Speakers Bureau; AMGEN: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Research Funding; CELGENE: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Research Funding, Speakers Bureau; JANSSEN: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Research Funding; TAKEDA: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Research Funding; THE BINDING SITE: Consultancy, Honoraria. Zavisic:Oncopeptides AB: Current Employment, Current equity holder in publicly-traded company. Thuresson:Oncopeptides: Consultancy, Current equity holder in publicly-traded company; Statisticon: Current Employment. Harmenberg:Oncopeptides AB: Consultancy, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Medivir AB: Current equity holder in publicly-traded company; Ultupharma AB: Current equity holder in private company. Richardson:Celgene/BMS, Oncopeptides, Takeda, Karyopharm: Research Funding.
This is an indirect comparison of clinical studies, including a phase 2 investigational study of melflufen in RRMM
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal